FDA Crackdown On Ozempic Copies: Supply Shortages Loom
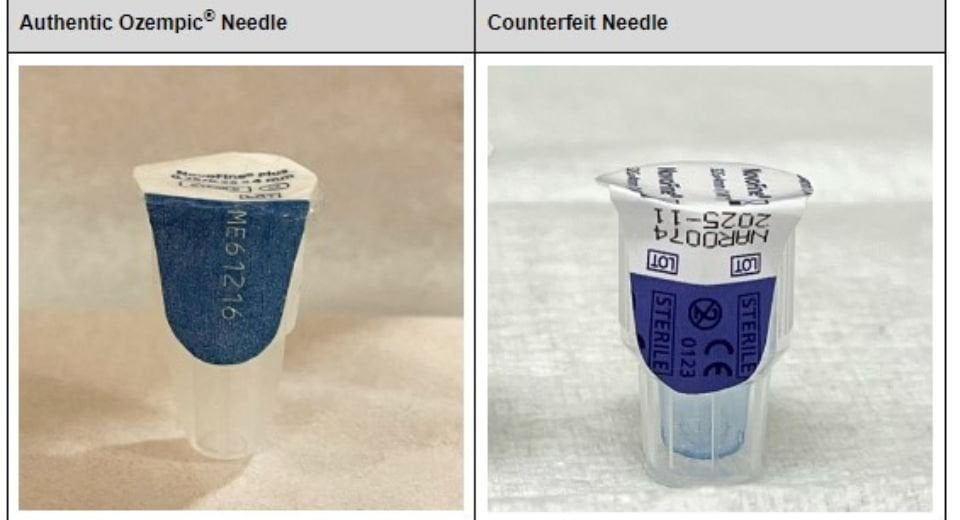
Table of Contents
Ozempic, a glucagon-like peptide-1 (GLP-1) receptor agonist, works by mimicking a natural hormone that regulates blood sugar levels and appetite. While primarily prescribed for type 2 diabetes, its efficacy in weight loss has led to a dramatic increase in demand, far outpacing supply. This heightened popularity has unfortunately attracted the attention of unscrupulous individuals and organizations producing and distributing counterfeit versions of the drug.
The FDA's Increased Scrutiny of Unauthorized Ozempic Copies
The FDA holds the critical responsibility of ensuring the safety and efficacy of all pharmaceuticals sold within the United States. The proliferation of counterfeit Ozempic poses a serious threat, not only to the integrity of the pharmaceutical market but also to the health and well-being of patients. The FDA's concern regarding counterfeit medications is substantial due to the inherent risks associated with these unauthorized copies.
The FDA is actively cracking down on the manufacturers and distributors of these counterfeit drugs. Their actions include:
- Increased inspections of manufacturing facilities: The FDA is ramping up inspections to identify and shut down illegal manufacturing operations producing counterfeit Ozempic.
- Issuance of warning letters to companies: Companies suspected of involvement in the distribution of counterfeit Ozempic are receiving warning letters, outlining the violations and demanding immediate cessation of their activities.
- Seizure of counterfeit products: The FDA has already seized significant quantities of counterfeit Ozempic, disrupting the illegal supply chain and removing dangerous products from the market.
- Public awareness campaigns on identifying counterfeit medications: The FDA is actively educating the public on how to identify counterfeit medications, emphasizing the importance of purchasing drugs from legitimate sources.
The health risks associated with using counterfeit Ozempic are substantial. These unauthorized copies may contain incorrect dosages, impure ingredients, or be entirely ineffective, potentially leading to serious health complications or treatment failures. Furthermore, the lack of quality control in these illegitimate operations creates an unpredictable risk profile for patients.
Impact on Ozempic Supply and Accessibility
The FDA's crackdown on unauthorized Ozempic copies is having a direct and significant impact on the legitimate supply of the medication. This increased regulatory scrutiny, coupled with the already high demand, is creating a perfect storm of supply chain issues.
Patients relying on Ozempic to manage their diabetes or weight are facing increasing challenges:
- Increased waiting lists for prescriptions: Many patients are experiencing extended waiting times to obtain their prescriptions.
- Difficulty finding Ozempic in pharmacies: Pharmacies are frequently running out of stock, leaving patients struggling to find the medication they need.
- Rising prices due to decreased supply: The limited supply is driving up the price of Ozempic, making it less accessible to many patients.
- Potential rationing of Ozempic by healthcare providers: Healthcare providers may be forced to ration Ozempic, prioritizing patients with the most critical needs.
These challenges underscore the urgent need for solutions to address the dwindling Ozempic supply and ensure continued access for patients who rely on this medication. Exploring alternative treatments and management strategies becomes crucial in mitigating the impact of these shortages.
Strategies to Combat Counterfeit Ozempic and Ensure Supply
Combating the counterfeit Ozempic problem and ensuring a stable supply requires a multi-faceted approach involving pharmaceutical companies, healthcare providers, regulatory bodies, and patients themselves.
- Improved packaging with anti-counterfeiting measures: Pharmaceutical companies are investing in enhanced packaging technologies, incorporating features like unique serial numbers, holograms, and tamper-evident seals to deter counterfeiting.
- Blockchain technology for supply chain tracking: Blockchain technology can provide a transparent and secure way to track the movement of Ozempic throughout the supply chain, making it more difficult for counterfeiters to infiltrate.
- Enhanced pharmacist training on identifying counterfeit drugs: Pharmacists play a critical role in identifying counterfeit medications. Increased training and awareness programs can equip them with the skills to detect suspicious products.
- Increased public awareness campaigns on drug safety: Public awareness campaigns educating patients on how to identify and avoid counterfeit medications are crucial in protecting patient safety.
Government initiatives are also essential in strengthening regulatory oversight and combating the trade in counterfeit pharmaceuticals. Increased funding for regulatory agencies, tougher penalties for counterfeiters, and international collaborations are all critical steps towards ensuring the integrity of the pharmaceutical supply chain.
Navigating the Ozempic Shortage and FDA Crackdown
The FDA's crackdown on counterfeit Ozempic is essential for protecting patient safety, but it has inadvertently exacerbated existing supply chain issues, resulting in shortages and accessibility challenges. The impact on patients relying on Ozempic for diabetes management or weight loss is significant, highlighting the urgent need for comprehensive strategies.
To navigate this challenging situation, patients should prioritize obtaining Ozempic from reputable pharmacies and healthcare providers. Learning to identify counterfeit medications through official FDA resources is critical. If facing shortages, patients should consult with their healthcare providers to explore alternative treatment options and management strategies. Reporting any suspicious activity regarding the sale or distribution of counterfeit Ozempic to the relevant authorities is also crucial. Understanding the complexities of the Ozempic supply chain and the implications of FDA regulation of Ozempic is crucial in safeguarding personal health. Be aware of the counterfeit Ozempic risks and act responsibly to protect yourself.

Featured Posts
-
 Ea Fc 24 Fut Birthday A Comprehensive Player Tier List
May 22, 2025
Ea Fc 24 Fut Birthday A Comprehensive Player Tier List
May 22, 2025 -
 Former Liverpool Managers Impact On Hout Bay Fc
May 22, 2025
Former Liverpool Managers Impact On Hout Bay Fc
May 22, 2025 -
 Optimalisatie Van Uw Verkoopprogramma Voor Abn Amro Kamerbrief Certificaten
May 22, 2025
Optimalisatie Van Uw Verkoopprogramma Voor Abn Amro Kamerbrief Certificaten
May 22, 2025 -
 Trans Australia Run Record Attempt On The Horizon
May 22, 2025
Trans Australia Run Record Attempt On The Horizon
May 22, 2025 -
 Prica S Reddita Postaje Film Sa Sydney Sweeney
May 22, 2025
Prica S Reddita Postaje Film Sa Sydney Sweeney
May 22, 2025
Latest Posts
-
 Route 15 On Ramp Closure Following Accident
May 22, 2025
Route 15 On Ramp Closure Following Accident
May 22, 2025 -
 Recent Drop In Virginia Gas Prices Gas Buddy Data Analysis
May 22, 2025
Recent Drop In Virginia Gas Prices Gas Buddy Data Analysis
May 22, 2025 -
 Lower Gas Prices In Virginia Gas Buddys Latest Report
May 22, 2025
Lower Gas Prices In Virginia Gas Buddys Latest Report
May 22, 2025 -
 Gas Buddy Update Average Gasoline Prices Fall In Virginia
May 22, 2025
Gas Buddy Update Average Gasoline Prices Fall In Virginia
May 22, 2025 -
 Philadelphia Market Sees Consistent Gas Price Increase 6 Cents Average
May 22, 2025
Philadelphia Market Sees Consistent Gas Price Increase 6 Cents Average
May 22, 2025
