Understanding The Explosive Growth Of The Vaccine Packaging Market

Table of Contents
Factors Driving the Growth of the Vaccine Packaging Market
Several key factors contribute to the remarkable expansion of the vaccine packaging market. Understanding these drivers is crucial for stakeholders across the pharmaceutical industry.
Increasing Global Vaccination Rates
The global push for higher vaccination coverage is a major catalyst. Increased awareness of preventable diseases, coupled with government initiatives promoting widespread vaccination programs, has significantly boosted demand for vaccines worldwide.
- Successful vaccination campaigns against diseases like polio and measles have demonstrated the effectiveness of large-scale immunization.
- The World Health Organization (WHO) reports a steady increase in global vaccination coverage, although challenges remain in achieving universal access.
- Outbreaks of infectious diseases, such as the recent COVID-19 pandemic, dramatically increase the demand for vaccines and highlight the critical need for reliable vaccine supply chains and robust vaccine packaging solutions.
Emergence of Novel Vaccines and Technologies
Advancements in vaccine technology, particularly the development of mRNA vaccines, have introduced new packaging challenges and opportunities. These novel vaccines often require specialized pharmaceutical packaging to maintain their stability and efficacy.
- mRNA vaccines, for example, are highly sensitive to temperature fluctuations and require ultra-low temperature cold chain packaging.
- Innovative packaging solutions, including temperature-controlled shippers and smart packaging incorporating sensors, are being developed to meet these demanding requirements.
- The transition to new vaccine types presents a considerable challenge, requiring continuous innovation in vaccine vial packaging and other packaging formats to ensure product stability and efficacy.
Stringent Regulatory Requirements and Compliance
The pharmaceutical industry is heavily regulated, and vaccine packaging is no exception. Strict adherence to safety and quality standards is paramount to ensure vaccine integrity and patient safety.
- Regulatory bodies like the FDA (Food and Drug Administration) in the US and the EMA (European Medicines Agency) in Europe set stringent guidelines for vaccine packaging materials and manufacturing processes.
- Compliance with Good Manufacturing Practices (GMP) is crucial, impacting the design, materials, and testing of all vaccine packaging components.
- Meeting these regulations can increase the cost and complexity of vaccine packaging, but it is essential for maintaining product safety and public trust.
Types of Vaccine Packaging and Their Applications
Effective vaccine packaging encompasses a multi-layered approach, involving primary, secondary, and tertiary packaging components, each with a specific function.
Primary Packaging
Primary packaging refers to the container in direct contact with the vaccine. Common types include vials, syringes, and pre-filled syringes. The choice of material (glass or plastic) depends on several factors.
- Glass vials offer excellent barrier properties and are suitable for many vaccines, but they are fragile and can be more expensive.
- Plastic vials and syringes offer improved break resistance and cost-effectiveness, but careful material selection is crucial to ensure compatibility with the vaccine formulation and maintain sterility.
- The selection of primary vaccine packaging materials must consider factors such as barrier properties, chemical inertness, sterility, and ease of handling.
Secondary and Tertiary Packaging
Secondary packaging (cartons, trays) and tertiary packaging (pallets, shipping containers) provide additional protection during transportation and storage. This is particularly crucial for maintaining the cold chain.
- Cartons offer protection against physical damage and provide space for labeling and information.
- Pallets facilitate efficient handling and transport, especially for large quantities of vaccines.
- Temperature-sensitive packaging is essential to maintain the required temperature range throughout the supply chain, preventing vaccine degradation.
Cold Chain Packaging Solutions
Maintaining the cold chain is paramount for preserving vaccine potency. Various cold chain packaging technologies help ensure the vaccine remains within the specified temperature range.
- Ice packs provide temporary temperature control for short-distance transport.
- Refrigerants and phase-change materials offer more extended temperature stability.
- Temperature-controlled containers, often incorporating insulation and monitoring systems, are crucial for long-distance shipment and storage. Real-time temperature monitoring within cold chain packaging systems provides critical data to mitigate risk and maintain vaccine integrity.
Future Trends in the Vaccine Packaging Market
The vaccine packaging market is dynamic and constantly evolving. Several trends are shaping its future trajectory.
Sustainable Packaging Solutions
The demand for environmentally friendly packaging solutions is increasing. This necessitates a shift towards biodegradable and recyclable materials.
- Bio-based polymers and other sustainable materials are being explored as alternatives to traditional plastics.
- Reducing packaging waste and minimizing the environmental impact of vaccine packaging is a growing priority for the industry.
- Regulatory frameworks are evolving to encourage the adoption of sustainable packaging solutions.
Smart Packaging Technologies
Smart packaging technologies leverage sensors and data analytics to enhance vaccine tracking and monitoring.
- Temperature sensors integrated into packaging provide real-time temperature data, allowing for proactive intervention if temperature excursions occur.
- RFID (Radio-Frequency Identification) tags enable tracking and tracing of vaccine shipments, ensuring transparency and accountability throughout the supply chain.
- Data analytics derived from smart packaging solutions can improve vaccine distribution efficiency and reduce wastage.
Advanced Packaging Designs for Enhanced Stability and Safety
Ongoing research and development focus on creating packaging that better protects vaccines during transportation.
- Innovative designs that improve shock absorption and reduce the risk of damage are constantly being developed.
- Improvements in material science and packaging design lead to more robust and cost-effective solutions for vaccine protection.
- Investing in R&D in this area is critical for maintaining vaccine integrity across the supply chain.
Conclusion
The explosive growth of the vaccine packaging market is driven by several interconnected factors: rising vaccination rates, innovative vaccine technologies, and stringent regulatory compliance. Effective vaccine packaging, encompassing primary, secondary, tertiary, and cold chain solutions, is crucial for ensuring vaccine potency, efficacy, and safety. The future of vaccine packaging involves a strong focus on sustainability, smart technologies, and advanced design to further improve the efficiency, safety, and reliability of vaccine delivery. To stay informed about the latest advancements, explore industry resources, attend relevant conferences, and connect with leading suppliers of vaccine packaging materials and solutions.

Featured Posts
-
 The Countrys Rising Business Stars Locations To Watch
May 30, 2025
The Countrys Rising Business Stars Locations To Watch
May 30, 2025 -
 Trump Grants Clemency 26 Pardons Including Notorious Gang Leader
May 30, 2025
Trump Grants Clemency 26 Pardons Including Notorious Gang Leader
May 30, 2025 -
 March Rainfall A Step Towards Recovering From Water Deficit
May 30, 2025
March Rainfall A Step Towards Recovering From Water Deficit
May 30, 2025 -
 Visualiza Tu Asiento Con El Nuevo Venue Virtual De Ticketmaster
May 30, 2025
Visualiza Tu Asiento Con El Nuevo Venue Virtual De Ticketmaster
May 30, 2025 -
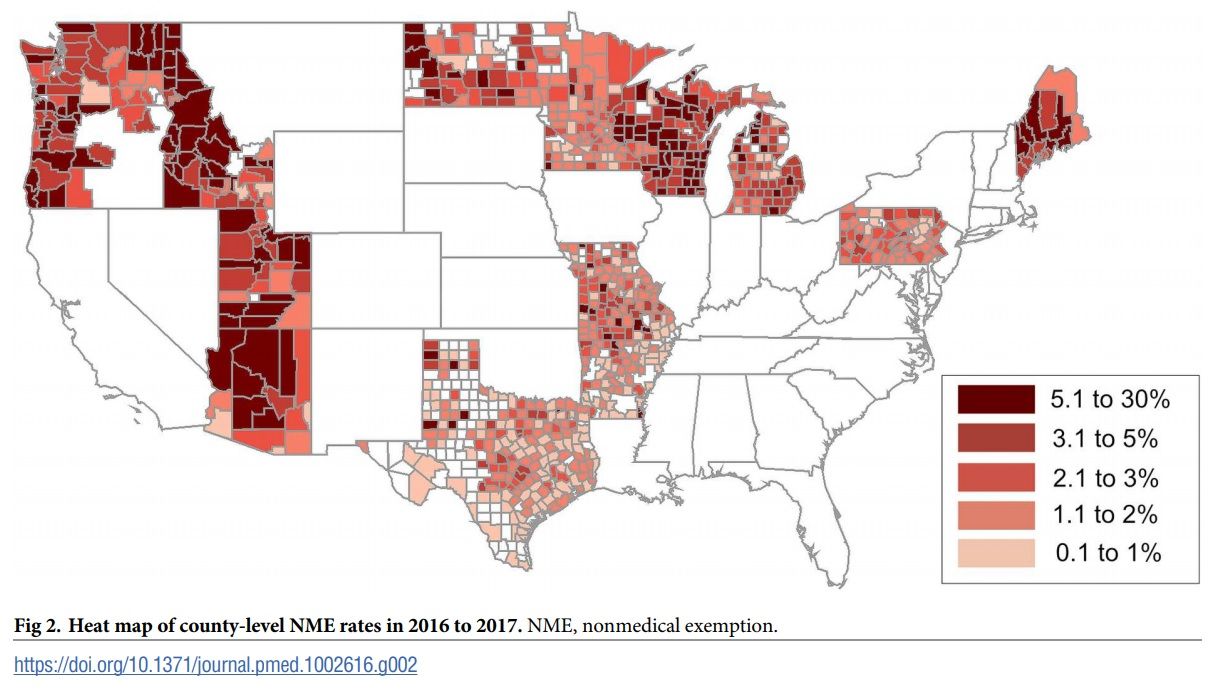 Measles In Texas A Growing Number Of Unconnected Outbreaks
May 30, 2025
Measles In Texas A Growing Number Of Unconnected Outbreaks
May 30, 2025
Latest Posts
-
 Alcaraz Through To Barcelona Open Round Of 16 Following Ruud
May 31, 2025
Alcaraz Through To Barcelona Open Round Of 16 Following Ruud
May 31, 2025 -
 Racial Abuse Case Beautician Receives No Jail Time
May 31, 2025
Racial Abuse Case Beautician Receives No Jail Time
May 31, 2025 -
 Musks Dogecoin Support No Regrets Over Trump Administration Involvement
May 31, 2025
Musks Dogecoin Support No Regrets Over Trump Administration Involvement
May 31, 2025 -
 Elon Musks Cost Cutting 101 Million In Dei Spending And 8 Million On Transgender Mice Eliminated
May 31, 2025
Elon Musks Cost Cutting 101 Million In Dei Spending And 8 Million On Transgender Mice Eliminated
May 31, 2025 -
 Elon Musks Pressure Campaign Did Trumps Team Block An Open Ai Uae Deal
May 31, 2025
Elon Musks Pressure Campaign Did Trumps Team Block An Open Ai Uae Deal
May 31, 2025
