Advanced CRISPR Technology: Achieving Greater Precision In Gene Modification
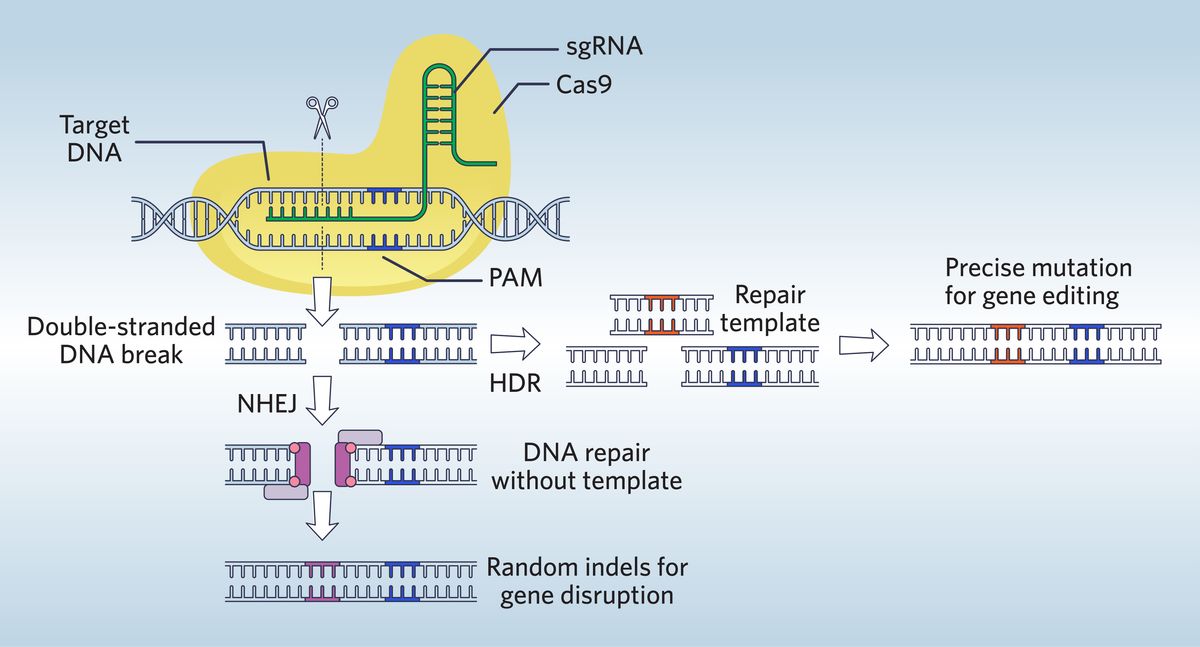
Table of Contents
Enhanced Specificity in CRISPR-Cas Systems
The accuracy of CRISPR-Cas9 hinges on the specificity of the Cas enzyme in recognizing its target DNA sequence. Early versions suffered from off-target effects, leading to unintended mutations. Fortunately, significant advancements have dramatically improved target specificity.
- Improved Cas Enzymes: Researchers have engineered variants of Cas9 and developed entirely new Cas enzymes with enhanced specificity. This includes:
- Base editors: These cleverly modified Cas enzymes can make precise single-base changes without causing double-strand breaks, minimizing off-target effects. They are particularly useful for correcting point mutations, the most common type of genetic variation causing disease.
- Prime editors: Offering even greater precision, prime editors combine a reverse transcriptase with a modified Cas enzyme. This allows for targeted insertion, deletion, or replacement of DNA sequences with high fidelity.
- Cas12a (Cpf1): This enzyme offers smaller size and different target recognition compared to Cas9, potentially enhancing specificity and reducing off-target effects.
- Cas13a: This RNA-targeting enzyme provides a promising tool for manipulating RNA, expanding the range of applications beyond DNA editing.
The mechanisms of these enzymes differ, but all aim to improve target recognition and reduce unwanted edits. This improved precision is crucial for therapeutic applications where unintended mutations could have severe consequences.
- Computational Tools: Computational tools and algorithms play an increasingly important role in optimizing CRISPR-Cas systems. These tools help predict potential off-target sites and design guide RNAs that maximize on-target activity while minimizing off-target effects. This computational design is crucial for ensuring the safety and efficacy of gene editing applications. These algorithms continuously evolve, further enhancing the precision of target site selection and minimizing off-target issues.
Advanced Delivery Methods for CRISPR Systems
Delivering CRISPR components (Cas enzyme and guide RNA) efficiently and specifically to target cells and tissues is a major bottleneck in gene editing. The effectiveness of gene editing directly correlates with the delivery method's efficiency.
-
Viral Vectors: Viral vectors, such as adeno-associated viruses (AAV) and lentiviruses, have been widely used for CRISPR delivery. AAVs are particularly attractive due to their relatively low immunogenicity and ability to transduce many cell types, while lentiviruses offer the advantage of integrating into the host genome. However, viral vectors have limitations, including packaging capacity and potential immunogenicity.
-
Non-Viral Methods: Non-viral delivery methods, including lipid nanoparticles (LNPs) and electroporation, offer alternatives to viral vectors. LNPs can encapsulate CRISPR components, protecting them from degradation and facilitating cellular uptake. Electroporation creates temporary pores in cell membranes to allow entry of the CRISPR components. Each method presents a unique set of advantages and disadvantages, impacting the efficiency and specificity of gene editing.
-
Emerging Delivery Systems: Ongoing research is focused on developing improved delivery systems, including targeted nanoparticles and novel methods to enhance specificity and efficacy. These advancements promise to significantly improve the delivery of CRISPR components to specific tissues and cell types, maximizing the precision of gene modification.
Multiplexed CRISPR for Complex Genome Engineering
Many genetic disorders result from the interplay of multiple genes. To address these complex diseases, the ability to simultaneously target multiple genes is crucial. Multiplexed CRISPR allows for the precise modification of multiple genomic loci simultaneously.
-
Strategies for Multiplexing: Several strategies exist for multiplexing CRISPR, including:
- Employing multiple guide RNAs targeting different genes with a single Cas enzyme.
- Utilizing different Cas enzymes, each with its own guide RNA targeting a unique site.
- Combining both approaches for more extensive genome engineering.
-
Applications: Multiplexed CRISPR has vast applications in both fundamental research and translational medicine. It has been used to model complex diseases in cell lines and animal models, and its potential in gene therapy for complex genetic disorders is immense. Advances are constantly being made in controlling the efficiency and precision of multiplexing to minimize off-target effects, even when targeting multiple loci simultaneously. This capability is essential for tackling complex biological systems and engineering novel genetic circuits.
Monitoring and Controlling CRISPR-Mediated Gene Editing
Accurate and efficient monitoring of gene editing outcomes is paramount to ensure the safety and efficacy of CRISPR-based therapies and research applications. This requires robust methods to assess both the efficiency of editing and the presence of off-target effects.
-
Monitoring Techniques: Several techniques are employed for monitoring gene editing:
- Next-Generation Sequencing (NGS): NGS provides comprehensive genome-wide analysis, allowing for precise identification of both on-target and off-target modifications.
- PCR-based assays: These targeted assays are cost-effective and highly sensitive for monitoring specific edits.
- Fluorescence-based assays: Reporter systems enable the visualization of successful gene editing events within cells, offering a convenient way to assess the efficiency of modification.
-
Quality Control and Error Correction: Quality control procedures are essential to minimize the risk of introducing unintended mutations. These measures encompass stringent validation of the guide RNAs, careful monitoring of editing outcomes, and the implementation of error correction strategies where possible.
Conclusion: The Future of Precision Gene Editing with Advanced CRISPR Technology
Advanced CRISPR technologies have significantly advanced the field of gene editing by increasing precision and reducing off-target effects. Improvements in Cas enzyme specificity, development of refined delivery methods, and the ability to perform multiplexed editing are all crucial steps toward realizing the full therapeutic potential of genome engineering. Robust monitoring techniques and the implementation of stringent quality control measures are essential for ensuring the safe and effective application of these powerful technologies. The future of advanced CRISPR technology looks bright, with applications spanning diverse fields such as medicine, agriculture, and biotechnology. The potential to cure genetic diseases, develop disease-resistant crops, and engineer novel biological systems is immense. We encourage you to delve deeper into this exciting field by exploring research articles, attending relevant conferences, and following the latest advancements in advanced CRISPR technology, revolutionizing gene modification and gene therapy. Numerous research institutions and journals are dedicated to publishing the latest findings; actively seeking out these resources will keep you abreast of the constantly evolving landscape of this groundbreaking technology.

Featured Posts
-
 Programma Tileoptikon Metadoseon Kyriakis 4 5
May 30, 2025
Programma Tileoptikon Metadoseon Kyriakis 4 5
May 30, 2025 -
 Elon Musks Alleged Paternity Of Amber Heards Twins Evidence And Reactions
May 30, 2025
Elon Musks Alleged Paternity Of Amber Heards Twins Evidence And Reactions
May 30, 2025 -
 Trump Administration Rejects 3 Billion Sunnova Energy Loan
May 30, 2025
Trump Administration Rejects 3 Billion Sunnova Energy Loan
May 30, 2025 -
 Economic Uncertainty The Threat Of Higher Inflation And Unemployment
May 30, 2025
Economic Uncertainty The Threat Of Higher Inflation And Unemployment
May 30, 2025 -
 Is Jon Jones Fighting Nate Diaz Aspinall Fight Rumors Debunked
May 30, 2025
Is Jon Jones Fighting Nate Diaz Aspinall Fight Rumors Debunked
May 30, 2025
Latest Posts
-
 Bmw Open 2025 Quarter Finals Zverev Vs Griekspoor A Munich Highlight
May 31, 2025
Bmw Open 2025 Quarter Finals Zverev Vs Griekspoor A Munich Highlight
May 31, 2025 -
 May Day In Kingston Images From A Robust Rally Daily Freeman
May 31, 2025
May Day In Kingston Images From A Robust Rally Daily Freeman
May 31, 2025 -
 Bmw Open 2025 Zverev Griekspoor Quarter Final Showdown In Munich
May 31, 2025
Bmw Open 2025 Zverev Griekspoor Quarter Final Showdown In Munich
May 31, 2025 -
 Madrid Open 2024 Berrettini Loses To Giron Despite Comeback Attempt
May 31, 2025
Madrid Open 2024 Berrettini Loses To Giron Despite Comeback Attempt
May 31, 2025 -
 Analysis Elon Musks Exit From The Trump Administration
May 31, 2025
Analysis Elon Musks Exit From The Trump Administration
May 31, 2025
